Nitrogen to Oxygen: Oxygen and nitrogen represent the main parts of air. All commercially available N2 and O2 produce as air. Nitrogen is a colourless, odourless and tasteless gas.
Nitrogen atoms have a powerful bond which makes nitrogen very stable, so it does not need to enter into chemical reactions. Due to these properties, nitrogen is in the food industry, steel processing, electronics and similar applications.
Oxygen also has no colour, smell or taste. Compared to nitrogen, oxygen reacts with most chemical elements.
Most living organisms require it for metabolic processes. It also accelerates fire, which is valid for the steel and glass industry.
Nitrogen to Oxygen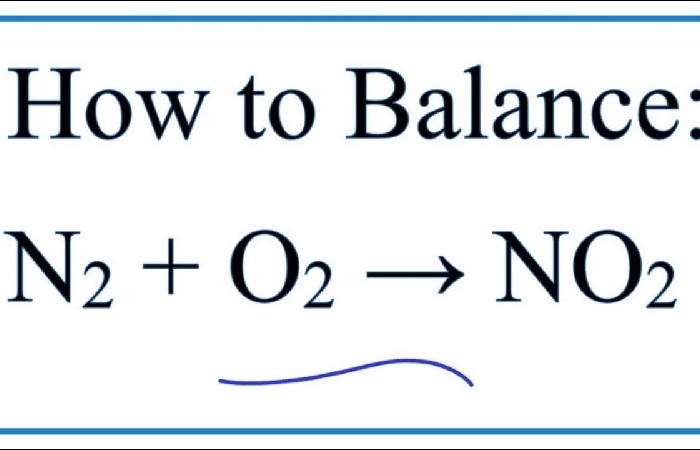
How does nitrogen produce oxygen?
The most common commercial method of producing oxygen is separation from the air using a cryogenic distillation process or a vacuum swing adsorption process. Nitrogen and argon get by separating from the air.
Can Nitrogen be Converted into Oxygen?
It is possible to convert existing nitrogen plants on pressure swing adsorption (PSA) to produce oxygen. By replacing the nitrogen production used by the carbon molecular sieve (CMS) with a molecular sieve of zeolite (ZMS) and other modifications, such as oxygen analyzers, control panel systems, flow valves etc.
The Reaction of Nitrogen with Oxygen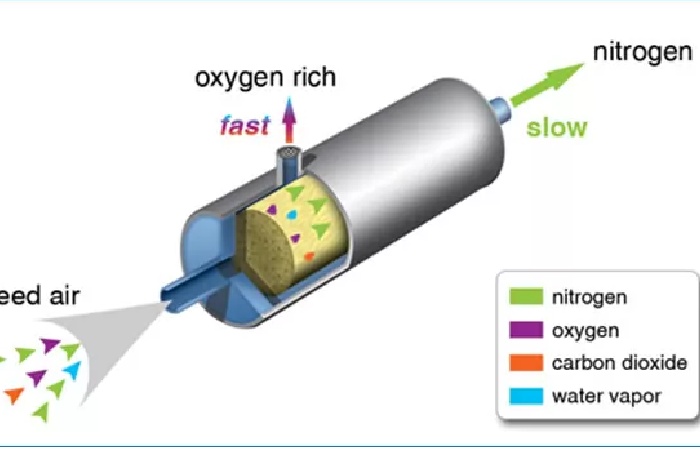
Oxygen is an essential element with atomic number 8. It is a transparent, odourless and colourless gas, blue in its liquid form.
Oxygen can also exist as a solid aggregate of blue crystals. It contains diatomic molecules.
Priestley observed the decomposition of mercuric oxide in an airtight apparatus. The scientist used a lens to direct the sun’s rays onto the rust. Curiously, Priestley did not realize that he had discovered oxygen and believed he had obtained a specific component from the air.
As for the interaction of nitrogen and oxygen, substances react in the presence of an electric current since nitrogen is a stable molecule and reacts reluctantly with other substances:
O₂ + N₂ = 2NO
Several nitrogen oxides range in oxidation state from one to five.
Several compounds come from a reaction between nitrogen and oxygen:
N₂O – nitrous oxide;
NO – nitric oxide;
N₂O₃ — dinitrogen trioxide;
NO₂ — nitrogen dioxide;
N₂O₅ — nitrogen pentoxide.
Click here to see an exciting experiment that consists of obtaining nitrogen dioxide and studying its properties.
Nitrous oxide, an anaesthetic, is by the degradation of ammonium nitrate. It is a colourless gas with a characteristic pleasant odour. The rust dissolves well in water.
N₂O is also a constant component of air. The process takes place at a temperature of 200°C. the reaction is:
NH₄NO₃ = 2Н₂О + N₂O↑
Nitric oxide, NO, is a colourless gas almost insoluble in water. This compound does not release oxygen but is known for addition reactions, such as with the toxic green-yellow chlorine gas:
2NO + Cl₂ = 2NOCl.
Uses of Nitrogen to Oxygen
Oxygen
The main consumer of oxygen is the steel industry. It is also to manufacture other metals, including copper and lead. It is more economical to use pure oxygen or oxygen-enriched air rather than air, as it increases reaction rates and allows the use of smaller chemical plants. In addition, it makes it easier to ensure that gases such as sulfur dioxide are not lost and pollute the atmosphere.
Among its other uses is the combustion of the carbon deposited in the fluid catalyst used in the catalytic cracking of diesel fuel.
Increasing use of oxygen in the treatment of wastewater and effluent from the industry. Polluted rivers and lakes get clean by dissolving oxygen gas directly into the water to promote a better ecological balance.
Along with sodium hydroxide, bleach paper pulp is an alternative to chlorine dioxide or sodium(I) chlorate (sodium hypochlorite).
Nitrogen
Nitrogen is essential to make ammonia. It is to purge pipes before welding (e.g. oil pipes) to ensure no flammable vapours remain. It is also extensively used to provide a static atmosphere; a process is known as “blanketing”, primarily to exclude oxygen. For example, nitrogen is helpful in food packaging, glass manufacturing, and semiconductor manufacturing.
Liquid nitrogen is to refrigerate food during transport. Medical specimens containing, for example, blood, vaccine viruses and semen can store for long periods if kept cold in liquid nitrogen.


![Nitrogen to Oxygen: Nitrogen into Oxygen [2024]](https://www.treasurebiz.com/wp-content/uploads/2022/06/Nitrogen-to-Oxygen-1200x675.jpg)
Review Nitrogen to Oxygen: Nitrogen into Oxygen [2024].